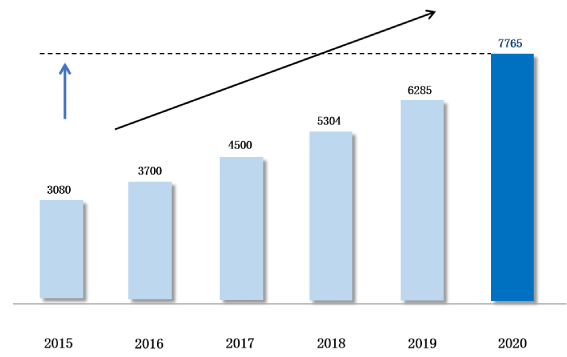
X 2.5
larger MD market
compared to that of 2015
1.023 Million
Healthcare Institutions
35,000
Hospitals
9.11 Million beds
in Healthcare Institutions
Favorable policies with administrative and institutional privileges are implemented in Hainan Free Trade Port and the Greater Bay Area as the pilot areas to further open the market by introducing more advanced medical devices to accelerate the industrial transformation.
Synchronized MD Market Access Mechanism
Regarding the marketed MDs with CE or FDA certification in HK or MO but without NMPA certificate, under the approval of local Guangdong provincial government as the precondition, the designated medical institutions in the Greater Bay Area may procure those mentioned MDs with clinical urgent need and clinical advancement which are currently used in the public hospitals of HK or MO.
Real-world Data Policy
As for the imported medical devices in clinical urgent need, real world data can be used for NMPA registration.
Hainan Free Trade Port
Favorable Policy
Real-world Data Policy [- Imported MDs - ]
Similar to that of the Greater Bay Area, regarding the imported medical devices in clinical urgent need, real world data can be used for NMPA registration as well.
Tariff & Tax Policy [ - JV Domestically Produced MDs - ]
Import tariff can somehow be exempted and tax can be levied in a favorable way when goods produced by the encouraged industrial enterprises enter the mainland China through the “second line”, which indicates the HFTP to other regions.
Start Business in CHINA
Various investment support from Fosun Pharma are available to help manufacturers adjust their business strategy with flexibility
Investment i n CHINA
Sole Proprietorship

Consultancy Service
CHINDEX Offers
Joint Venture

Financial Support

Human Resource

Land Resource
CHINDEX & FOSUN PHARMA Offer
Unfortunately, it is NOT legal unless the manufacturers have branches with qualification in China to deal with the registration issues. Otherwise, it is a must-do that manufacturers overseas are required to appoint a Chinese agent for the medical device registration.
Regarding the registration fee for NMPA, as for the imported medical devices, the registration of Class II medical device needs 210,900 CNY while that of Class III costs 308,800 CNY. In addition, other kinds of possible fees should be taken into consideration such as fees of document translation, clinical trial, and so forth.
After the registration materials are accepted by NMPA, technical review for Class II medical devices takes 60 working days to process while for Class III medical devices, technical review takes 90 working days. If supplementary materials are required by NMPA, manufacturers are obliged to conduct submission within one year, otherwise the review may result in termination.
MDs with CE or FDA certificate are feasible to enter SAR without NMPA certificate. As for the Greater Bay Area and Hainan province, MDs marketed in HK or MO as a premise can enter the pilot areas if designated medical institutions in pilot areas are willing to procure medical devices out of clinical urgent needs under the approval of local government.
Copyright © Chindex Medical Limited